In terms of the enthalpy of formation,which of the following compounds is the most unstable compared to its elements under standard conditions?
A) 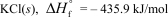
B) 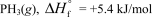
C) 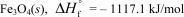
D) 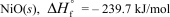
E) 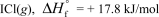
Correct Answer:
Verified
Q85: Which statement regarding combustion of a sample
Q107: Fuel value is _
A)the cost of energy
Q113: When a 13.0 g sample of NaOH(s)dissolves
Q114: Isooctane is a good model for gasoline.When
Q115: In an experiment,100.0 g of water at
Q116: When 1.14 g of octane (molar mass
Q117: Given the following reactions,what is the
Q120: A food sample was burned in a
Q121: The complete combustion of carbon compounds
Q122: Coal is converted into cleaner,more transportable fuels
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents