Most integrated circuits are made from silicon,which can be obtained from an inexpensive starting material,SiO2.One step in the purification of silicon is to separate it from solid impurities by forming silicon tetrachloride gas.Given the following reactions,what is the overall enthalpy change in converting 1.00 mol of silicon dioxide into pure silicon?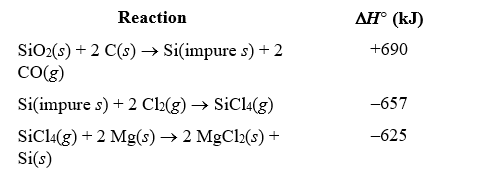
A) +1972 kJ
B) -1972 kJ
C) -592 kJ
D) +592 kJ
E) -625 kJ
Correct Answer:
Verified
Q100: A 65.0 g piece of chromium [cp
Q101: In an experiment,20.0 g of ice
Q102: Indicate which of the following is NOT
Q103: Given the following reactions,what is the
Q104: Given the following reactions,estimate the overall
Q106: In an experiment,110.0 g of iron is
Q107: Use the following information to determine
Q108: Nitroglycerin decomposes to form carbon dioxide,water
Q109: When 7.29 g hydrochloric acid (36.46 g/mol)and
Q110: The freezing point of ammonia (NH3,17.04 g/mol)is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents