The combustion of butane (C4H10) forms carbon dioxide and water.What is the stoichiometric coefficient for oxygen in the balanced equation when 1 mol of butane undergoes combustion?
A) 9
B) 13
C) 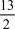
D) 
E) 5
Correct Answer:
Verified
Q1: A sample of water (H2O) contains 1.81
Q14: Which one of the following samples contains
Q20: Hydrogen peroxide decomposes to produce water
Q24: Identify the set of stoichiometric coefficients
Q25: In a one type of fuel
Q26: Sulfuric acid reacts with a vanadium
Q27: Fe2O3(s) and powdered aluminum can react with
Q27: Extracting copper from sulfide ores can
Q30: During a fermentation process, sucrose (C12H22O11) can
Q43: The average adult exhales about 1.0 kg
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents