In a one type of fuel cell,methanol (CH3OH) enters on one side of the unit,and air enters on the other side.Both circulate past electrodes and chemically react to produce carbon dioxide and water along with electricity.Which chemical equation best represents the reaction that occurs?
A) CH3OH 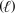 CO2(g) + 2 H2O
CO2(g) + 2 H2O 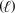
B) 2 CH3OH 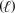 + 3 O2(g) 2 CO2(g) + 4H2O
+ 3 O2(g) 2 CO2(g) + 4H2O 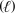
C) CH3OH 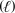 CH2(g) + H2O
CH2(g) + H2O 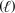
D) CH3OH 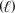 + N2(g) CH3(g) + NO(g)
+ N2(g) CH3(g) + NO(g)
E) CH3OH 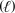 + O2(g) CO2(g) + H2O
+ O2(g) CO2(g) + H2O 
Correct Answer:
Verified
Q4: Air bags in cars inflate when an
Q20: Hydrogen peroxide decomposes to produce water
Q22: The combustion of butane (C4H10)forms carbon dioxide
Q24: Identify the set of stoichiometric coefficients
Q26: Sulfuric acid reacts with a vanadium
Q27: Extracting copper from sulfide ores can
Q29: Which statement,A-D,regarding photosynthesis is NOT correct? In
Q30: During a fermentation process, sucrose (C12H22O11) can
Q30: Chlorine atoms in the atmosphere can
Q43: The average adult exhales about 1.0 kg
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents