If 98.0 g B2O3 (69.62 g/mol) and 125 g HF (20.01 g/mol) are combined and allowed to react according to the given equation,what mass of the excess reactant will be left over? B2O3(s) + 6 HF(aq) 2 BF3(g) + 3 H2O 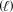
A) 72.5 g B2O3 will be left over.
B) 25.5 g B2O3 will be left over.
C) 28.2 g HF will be left over.
D) 7.34 g HF will be left over.
E) Both are completely comsumed.
Correct Answer:
Verified
Q101: Copper metal reacts with nitric acid to
Q102: Mathematically combine the following three reactions
Q103: Ammonia, NH3, is industrially produced from N2
Q103: A mass of 11.60 g of phosphoric
Q105: Write the balanced reaction equation for the
Q107: Solid nickel(II)phosphate and aqueous sodium chloride are
Q108: Gold metal will dissolve in an
Q109: Household bleach contains 5.0% sodium hypochlorite
Q111: A 2.0 kg bottle of sodium
Q139: Write a definition of the phrase "stoichiometry
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents