Gold metal will dissolve in an aqueous sodium cyanide solution in the presence of O2.Balance the equation showing this process.
______Au(s)+ ______NaCN(aq)+ ______O2(g)+ ______H2O 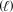 ______NaAu(CN)2(aq)+ ______NaOH(aq)
______NaAu(CN)2(aq)+ ______NaOH(aq)
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q103: Ammonia, NH3, is industrially produced from N2
Q103: A mass of 11.60 g of phosphoric
Q105: Write the balanced reaction equation for the
Q106: If 98.0 g B2O3 (69.62 g/mol)and
Q107: Solid nickel(II)phosphate and aqueous sodium chloride are
Q109: Household bleach contains 5.0% sodium hypochlorite
Q111: A 2.0 kg bottle of sodium
Q111: A copper ore consisting of 12.5%
Q113: Quicklime,CaO,can be produced by heating calcium hydroxide,Ca(OH)2.What
Q139: Write a definition of the phrase "stoichiometry
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents