Which one of the following statements is NOT correct about CO,CO+,and CO-? Use the following MO diagram. 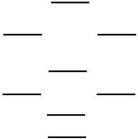
A) All have at least 6 electrons in bonding MOs.
B) CO has the shortest bond.
C) All have a permanent dipole.
D) CO is paramagnetic.
E) Two of these have the same bond order.
Correct Answer:
Verified
Q111: There are two possible structures for 1,2-dichloroethylene,
Q123: Draw the Lewis structure of ClCN.Identify
Q128: Draw the Lewis structure of Cl2O.Give the
Q135: Draw the Lewis structure of BrF5.Give the
Q137: According to the molecular orbital energy level
Q138: Boron nitride,BN,is a new high-tech material.According to
Q140: ICl3 and SO3 have one central atom.Do
Q142: Identify the molecular structure of the molecular
Q143: The amide functional group is the fundamental
Q177: For which one of the following molecules
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents