Boron nitride,BN,is a new high-tech material.According to the molecular orbital energy level diagram below,which one of the following statements is NOT correct about boron nitride? 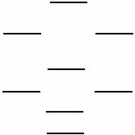
A) The bond order in BN is 2.0.
B) The cation BN+ has a longer bond than BN.
C) BN is paramagnetic.
D) The bond order in the cation BN+ is 1.5.
E) The anion BN- has a weaker bond than BN.
Correct Answer:
Verified
Q111: There are two possible structures for 1,2-dichloroethylene,
Q112: Draw the Lewis structure with the lowest
Q123: Draw the Lewis structure of ClCN.Identify
Q128: Draw the Lewis structure of Cl2O.Give the
Q137: According to the molecular orbital energy level
Q140: Which one of the following statements is
Q142: Identify the molecular structure of the molecular
Q143: The amide functional group is the fundamental
Q175: Of the following molecules (O3, SCl2,
Q177: For which one of the following molecules
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents