The heat of formation of Fe2O3(s) is -826.0 kJ/mol.Calculate the heat of the reaction 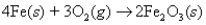 when a 53.99-g sample of iron is reacted.
when a 53.99-g sample of iron is reacted.
A) -199.6 kJ
B) -399.2 kJ
C) -798.5 kJ
D) -1597 kJ
E) -2.230 104 kJ
Correct Answer:
Verified
Q68: The _ of a system is the
Q69: The heat combustion of acetylene,C2H2(g),at 25°C
Q70: The coal with the highest energy available
Q71: Using the following thermochemical data,calculate
Q72: All of the following statements about the
Q74: Using the following thermochemical data:
2Cr(s)+
Q75: The following statements concerning petroleum are all
Q76: One of the main advantages of hydrogen
Q77: Using the information below,calculate
Q78: For the reaction: AgI(s)+ 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents