For the reaction: AgI(s) + 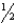 Br2(g) AgBr(s) +
Br2(g) AgBr(s) + 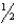 I2(s) , H° = -54.0 kJ
I2(s) , H° = -54.0 kJ
Hf° for AgBr(s) = -100.4 kJ/mol
Hf° for Br2(g) = +30.9 kJ/mol
The value of Hf° for AgI(s) is:
A) -123.5 kJ/mol
B) +77.3 kJ/mol
C) +61.8 kJ/mol
D) -77.3 kJ/mol
E) -61.8 kJ/mol
Correct Answer:
Verified
Q73: The heat of formation of Fe2O3(s)is
Q74: Using the following thermochemical data:
2Cr(s)+
Q75: The following statements concerning petroleum are all
Q76: One of the main advantages of hydrogen
Q77: Using the information below,calculate
Q79: Which of the following is not being
Q80: The combustion of hydrogen gas releases 286
Q81: Consider the following data: Q82: Consider the following reaction: Q83: Acetylene (C2H2)and butane (C4H10)are gaseous fuels.Determine the![]()
2Al(s)+ 3Cl2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents