A sample of oxygen gas has a volume of 1.72 L at 27°C and 800.0 torr.How many oxygen molecules does it contain?
A) 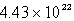
B) 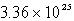
C) 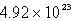
D) 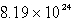
E) none of these
Correct Answer:
Verified
Q5: A sample of helium gas occupies 14.7
Q27: A 41.1-g sample of Ne gas exerts
Q28: Mercury vapor contains Hg atoms.What is the
Q29: Consider three 1-L flasks at STP.Flask A
Q30: A 3.60-L sample of carbon monoxide is
Q31: An automobile tire is filled with air
Q33: Three 1.00-L flasks at 25°C and 725
Q34: You are holding four identical balloons each
Q35: Three 1.00-L flasks at 25°C and 725
Q37: What volume is occupied by 21.0 g
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents