Gaseous 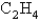 reacts with
reacts with 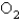 according to the following equation:
according to the following equation: 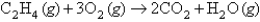 What volume of oxygen gas at STP is needed to react with 5.75 mol of
What volume of oxygen gas at STP is needed to react with 5.75 mol of 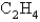 ?
?
A) 17.3 L
B) 42.9 L
C) 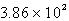 L
L
D) 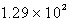 L
L
E) Not enough information is given to solve the problem.
Correct Answer:
Verified
Q41: A sample of gas is in a
Q42: Four identical 1.0-L flasks contain the gases
Q43: Which of the following is the best
Q44: It is found that 250.mL of a
Q45: Four identical 1.0-L flasks contain the gases
Q47: Four identical 1.0-L flasks contain the gases
Q48: Four identical 1.0-L flasks contain the gases
Q49: Given reaction 2NH3(g)+ 3Cl2(g)
Q50: When 0.72 g of a liquid is
Q51: You carry out the reaction represented
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents