A sample of gas is in a 50.0-mL container at a pressure of 645 torr and a temperature of 25°C.The entire sample is heated to a temperature of 35°C and transferred to a new container whose volume is 98.7 mL.The pressure of the gas in the second container is about:
A) 457 torr
B) 316 torr
C) 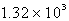 torr
torr
D) 65 torr
E) 338 torr
Correct Answer:
Verified
Q5: A sample of helium gas occupies 14.7
Q37: What volume is occupied by 21.0 g
Q38: Consider three 1-L flasks at STP.Flask A
Q39: A 7.94-g piece of solid CO2 (dry
Q40: Consider three 1-L flasks at STP.Flask A
Q42: Four identical 1.0-L flasks contain the gases
Q43: Which of the following is the best
Q44: It is found that 250.mL of a
Q45: Four identical 1.0-L flasks contain the gases
Q46: Gaseous ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents