A solid acid HX is mixed with water.Two possible solutions can be obtained.Which of the following is true? I. 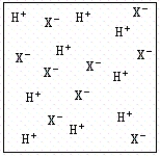 II.
II. 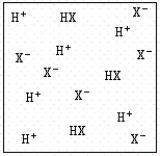
A) In case I,HX is acting like a weak acid,and in case II,HX is acting like a strong acid.
B) In case I,HX is acting like a strong acid,and in case II,HX is acting like a weak acid.
C) In both cases,HX is acting like a strong acid.
D) In both cases,HX is acting like a weak acid.
E) HX is not soluble in water.
Correct Answer:
Verified
Q3: Polar molecules have an unequal distribution of
Q4: How many grams of NaCl are contained
Q5: All of the following are weak acids
Q6: Consider two organic molecules,ethanol and benzene.One dissolves
Q7: The man who discovered the essential nature
Q9: What mass of calcium chloride,CaCl2,is needed to
Q10: A 74.28-g sample of Ba(OH)2 is dissolved
Q11: An unknown substance dissolves readily in water
Q12: An acid is a substance that produces
Q13: How many grams of NaOH are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents