Sulfamic acid,HSO3NH2 (molar mass = 97.1 g/mol) ,is a strong monoprotic acid that can be used to standardize a strong base: 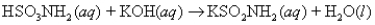 A 0.165-g sample of HSO3NH2 required 19.4 mL of an aqueous solution of KOH for a complete reaction.What is the molarity of the KOH solution?
A 0.165-g sample of HSO3NH2 required 19.4 mL of an aqueous solution of KOH for a complete reaction.What is the molarity of the KOH solution?
A) 0.00170 M
B) 8.76 M
C) 0.0876 M
D) 0.0330 M
E) none of these
Correct Answer:
Verified
Q62: You mix 55 mL of 1.00 M
Q63: An unknown diprotic acid requires 44.39 mL
Q64: When solutions of carbonic acid and aluminum
Q65: When solutions of carbonic acid and potassium
Q66: A 0.307-g sample of an unknown triprotic
Q68: When solutions of carbonic acid and copper(II)hydroxide
Q69: With what volume of 5.00 M HF
Q70: You have 88.6 mL of a 2.50
Q71: What mass of NaOH is required to
Q72: A 3.00-g sample of an alloy (containing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents