The following equation describes the oxidation of ethanol to acetic acid by potassium permanganate: 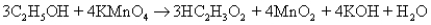 5.00 g of ethanol and an excess of aqueous KMnO4 are reacted,and 4.24 g of HC2H3O2 result.What is the percent yield?
5.00 g of ethanol and an excess of aqueous KMnO4 are reacted,and 4.24 g of HC2H3O2 result.What is the percent yield?
A) 100%
B) 65.1%
C) 21.7%
D) 34.9%
E) 4.24 g HC2H3O2 is impossible since it represents more than 100% yield.
Correct Answer:
Verified
Q115: The reaction of 11.9 g of CHCl3
Q116: The limiting reactant in a reaction
A)is the
Q117: How many grams of H2O will be
Q118: If 45.0 g of O2 are mixed
Q119: Which of the following statements is
Q121: Vitamin B12,cyanocobalamin,is essential for human nutrition.It's concentrated
Q122: The reactant which,when used up completely,can produce
Q123: The hormone epinephrine is released in the
Q124: The _ in a chemical equation represent
Q125: Naturally occurring iron contains 5.82% 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents