Naturally occurring iron contains 5.82% 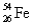 ,91.66%
,91.66% 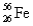 ,2.19%
,2.19% 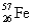 ,and 0.33%
,and 0.33% 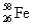 .The respective atomic masses are 53.940 amu,55.935 amu,56.935 amu,and 57.933 amu.Calculate the average atomic mass of iron.
.The respective atomic masses are 53.940 amu,55.935 amu,56.935 amu,and 57.933 amu.Calculate the average atomic mass of iron.
Correct Answer:
Verified
Q120: The following equation describes the oxidation of
Q121: Vitamin B12,cyanocobalamin,is essential for human nutrition.It's concentrated
Q122: The reactant which,when used up completely,can produce
Q123: The hormone epinephrine is released in the
Q124: The _ in a chemical equation represent
Q126: Give the empirical formula for the following
Q127: In a metallurgical process the mineral
Q128: The percent yield is a ratio of
Q129: The characteristic odor of pineapple is due
Q130: In order to determine the molecular formula
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents