The following question refers to a galvanic cell that utilizes the following reaction (unbalanced) : (AuCl4) -(aq) + Cu(s) Au(s) + Cl-(aq) + Cu2+(aq)
Given the following information,determine the standard cell potential:
Species
Standard Reduction Potential (V)
Au3+(aq)
1.4980
Cu2+(aq) 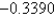
A) 1.1590 V
B) 1.8370 V
C) 3.8160 V
D) 0.8200 V
E) 4.1550 V
Correct Answer:
Verified
Q49: What is the cell reaction for the
Q50: How many electrons are transferred in the
Q51: What is the cell potential at 25°C
Q52: What is the balanced chemical equation
Q53: Consider an electrochemical cell with a
Q55: Which of the following is the best
Q56: Copper will spontaneously reduce which of the
Q57: The galvanic cell described by Zn(s)|
Q58: A cell is set up with
Q59: The following has a potential of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents