Which of the following statements is true concerning the electrochemical cell depicted below? Mg | Mg2+(aq) || Cu2+(aq) | Cu
Mg2+(aq) + 2e- Mg(s) ; 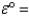 -2.38 V
-2.38 V
Cu2+(aq) + 2e- Cu(s) ; 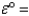 0.34 V
0.34 V
A) The cell reaction is spontaneous with a standard cell potential of 2.72 V.
B) The cell reaction is spontaneous with a standard cell potential of 2.04 V.
C) The cell reaction is nonspontaneous with a standard cell potential of -2.72 V.
D) The cell reaction is nonspontaneous with a standard cell potential of -2.04 V.
E) The cell is at equilibrium.
Correct Answer:
Verified
Q74: Tables of standard reduction potentials are
Q75: For a reaction in a voltaic
Q76: Consider an electrochemical cell with a
Q77: Consider an electrochemical cell with a
Q78: What is
Q80: Given: 2H+(aq)+ 2e-
Q81: A concentration cell is constructed using
Q82: An excess of finely divided iron
Q83: Which of the following statements is/are
Q84: The standard potential for the reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents