Consider an electrochemical cell with a zinc electrode immersed in 1.0 M Zn2+ and a nickel electrode immersed in 0.10 M Ni2+.
Zn2+ + 2e- Zn
° = -0.76 V
Ni2+ + 2e- Ni
° = -0.23 V
-Calculate at 25°C for the cell shown below,given the following data: 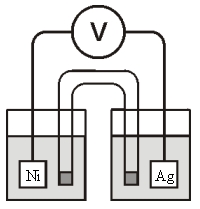
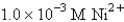
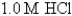
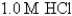
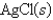
Ksp for AgCl = 1.6 10-10
A) 0.83 V
B) 0.54 V
C) 1.01 V
D) 2.98 V
E) cannot be determined from the data given
Correct Answer:
Verified
Q72: Under standard conditions,which of the following operations
Q73: A galvanic cell consists of
Q74: Tables of standard reduction potentials are
Q75: For a reaction in a voltaic
Q76: Consider an electrochemical cell with a
Q78: What is
Q79: Which of the following statements is
Q80: Given: 2H+(aq)+ 2e-
Q81: A concentration cell is constructed using
Q82: An excess of finely divided iron
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents