Consider the gas phase reaction NO + 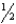 O2
O2  NO2 for which H° = -56.91 kJ and K = 1.46 106 at 25°C.
NO2 for which H° = -56.91 kJ and K = 1.46 106 at 25°C.
-Calculate K for the following reaction at 25°C: 2NO + O2  2NO2
2NO2
A) 2.92 106
B) 2.13 1012
C) 7.30 105
D) 1.21 103
E) 1.46 106
Correct Answer:
Verified
Q90: The reaction 2H2O(g)
Q91: For a particular reaction the equilibrium
Q92: The acid dissociation constant for a weak
Q93: Calculate
Q94: Consider the reaction 2NO2(g) 
Q96: What would be the effect on the
Q97: The standard molar free energies of
Q98: Given the following free energies of
Q99: For this system at equilibrium,how will raising
Q100: For the following reaction,CO2(g)+ 2H2O(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents