The acid dissociation constant for a weak acid HX at 25°C is 4.3 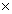 10-8.Calculate the free energy of formation for X-(aq) at 25°C.The standard free energies of HX(aq) and H+(aq) at 25°C are -208.3 kJ/mol and 0,respectively.
10-8.Calculate the free energy of formation for X-(aq) at 25°C.The standard free energies of HX(aq) and H+(aq) at 25°C are -208.3 kJ/mol and 0,respectively.
A) -205 kJ/mol
B) 250 kJ/mol
C) 0
D) -166 kJ/mol
E) -250 kJ/mol
Correct Answer:
Verified
Q87: The equilibrium constant K for the
Q88: The standard free energy of formation
Q89: Consider the following hypothetical reaction (at
Q90: The reaction 2H2O(g)
Q91: For a particular reaction the equilibrium
Q93: Calculate
Q94: Consider the reaction 2NO2(g) 
Q95: Consider the gas phase reaction NO
Q96: What would be the effect on the
Q97: The standard molar free energies of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents