The 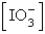 in a saturated solution of
in a saturated solution of 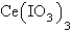 is 5.93 10-3 M.Calculate the Ksp for
is 5.93 10-3 M.Calculate the Ksp for 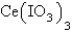 .
.
A) 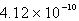
B) 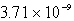
C) 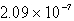
D) 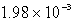
E) none of these
Correct Answer:
Verified
Q2: Calculate the concentration of chromate ion,CrO42-,in
Q3: The solubility of silver phosphate,Ag3PO4, at
Q4: Silver chromate,Ag2CrO4,has a Ksp of 8.96
Q5: The solubility of Cd(OH)2 in water
Q6: The molar solubility of PbI2 is
Q8: The correct mathematical expression for finding the
Q9: A 300.0-mL saturated solution of copper(II)peroidate,Cu(IO4)2,contains 0.30
Q10: An unknown salt,M2Z,has a Ksp of
Q11: Calculate the concentration of the silver
Q12: The solubility of an unknown salt,M3Z2, at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents