Calculate the concentration of the silver ion in a saturated solution of silver chloride,AgCl (Ksp = 1.57 10-10) .
A) 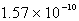
B) 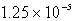
C) 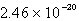
D) 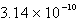
E) none of these
Correct Answer:
Verified
Q6: The molar solubility of PbI2 is
Q7: The Q8: The correct mathematical expression for finding the Q9: A 300.0-mL saturated solution of copper(II)peroidate,Cu(IO4)2,contains 0.30 Q10: An unknown salt,M2Z,has a Ksp of Q12: The solubility of an unknown salt,M3Z2, at Q13: The solubility of an unknown salt,MZ3, at Q14: The solubility in mol/L of Ag2CrO4 Q15: It is observed that 7.50 mmol of Q16: The solubility of CaSO4 in pure water![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents