How many moles of CaF2 will dissolve in 3.0 liters of 0.089 M NaF solution? (Ksp for CaF2 = 4.0 10-11)
A) 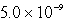
B) 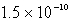
C) 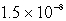
D) 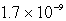
E) none of these
Correct Answer:
Verified
Q36: In a solution prepared by adding
Q37: The Ksp for PbF2 is 4.0
Q38: Calculate the concentration of Al3+ in
Q39: The molar solubility of BaCO3 (Ksp
Q40: The Ksp of AgI is 1.5
Q42: Which of the following solid salts is
Q43: What is the limiting reagent in the
Q44: What is the best way to ensure
Q45: Which of the following solid salts is
Q46: You have a solution consisting of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents