A solution containing 10.mmol of 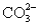 and 5.0 mmol of
and 5.0 mmol of 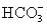 is titrated with 1.7 M HCl.What total volume of HCl must be added to reach the second equivalence point?
is titrated with 1.7 M HCl.What total volume of HCl must be added to reach the second equivalence point?
A) 8.8 mL
B) 5.9 mL
C) 2.9 mL
D) 14.7 mL
E) 19.7 mL
Correct Answer:
Verified
Q57: Consider the titration of 300.0 mL
Q58: What is the molarity of a sodium
Q59: If 25 mL of 0.750 M HCl
Q60: If 25.0 mL of 0.451 M NaOH
Q61: A solution contains 10.mmol of H3PO4
Q63: You have 100.0 mL of 0.100
Q64: A 50.00-mL solution of 0.0350 M
Q65: A 100.0-mL sample of 0.2 M
Q66: How many of the following will raise
Q67: Consider the following information about the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents