Consider the following reaction: 2HF(g)  H2(g) + F2(g) (K = 1.00
H2(g) + F2(g) (K = 1.00 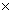 10-2)
10-2)
Given 1.00 mole of HF(g) ,0.362 mole of H2(g) ,and 0.750 mole of F2(g) are mixed in a 5.00 L flask,determine the reaction quotient,Q.
A) Q = 0.0543
B) Q = 0.272
C) Q = 0.0679
D) Q = 2.11
E) none of these
Correct Answer:
Verified
Q34: The reaction quotient for a system
Q35: For the reaction NO(g)+ Q36: Which of the following is true for Q37: A 10.0-g sample of solid NH4Cl is Q38: An equilibrium reaction,A2(g)+ 3B2(g) Q40: Which of the following statements about the Q41: Consider the following reaction (assume an ideal Q42: Raising the pressure by lowering the volume Q43: Carbon disulfide and chlorine react according to Q44: The reaction: H2(g)+ I2(g) ![]()

![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents