Consider the following rate law: 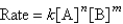 How are the exponents n and m determined?
How are the exponents n and m determined?
A) by using the balanced chemical equation
B) by using the subscripts for the chemical formulas
C) by using the coefficients of the chemical formulas
D) by educated guess
E) by experiment
Correct Answer:
Verified
Q2: Consider the reaction: Q3: The following questions refer to the Q4: A general reaction written as A Q5: A general reaction written as A Q6: Determine the initial rate of B Q7: What is the numerical value of the Q8: Determine the initial rate of C Q9: The following initial rate data were Q10: Consider the following data concerning the Q11: The average value for the rate![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents