For the reaction A Products,successive half-lives are observed to be 10.0 min and 40.0 min.
-The reaction follows the integrated rate law
A) 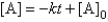
B) 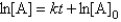
C) 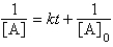
D) 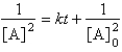
E) none of these
Correct Answer:
Verified
Q44: The reaction Q45: The reaction Q46: A chemical reaction that is first Q47: The OH· radical disproportionates according to Q48: Determine the magnitude of the pseudo-rate Q50: At a given temperature,a first-order reaction Q51: For the reaction A Q52: Consider the reaction 3A + B Q53: The reaction Q54: Consider the reaction 3A + B Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

