The radioactive nuclide 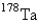 undergoes first-order decay with a half-life of 9.31 min.If a quantity of
undergoes first-order decay with a half-life of 9.31 min.If a quantity of 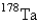
Is produced,what fraction remains after 82.5 seconds?
A) 0.113
B) 0.00215
C) 0.148
D) 0.903
E) 0.0973
Correct Answer:
Verified
Q60: Consider the reaction 3A + B
Q61: Two isomers (A and B)of a
Q62: The rate law for a reaction
Q63: A particular first-order reaction has a rate
Q64: The experimental rate law for the
Q66: Determine the molecularity of the following
Q67: Of what use is it to find
Q68: The reaction A
Q69: The reaction A
Q70: The decomposition of N2O5(g)to NO2(g)and O2(g)obeys
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents