The thermite reaction, shown below, is very exothermic and is often used as an initiator in fireworks. In this reaction, ________ is the reducing agent. 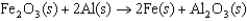
A) Fe2O3(s)
B) Al(s)
C) Fe(s)
D) Al2O3(s)
E) H2(g)
Correct Answer:
Verified
Q1: In the smelting of iron from iron
Q2: Sodium carbonate is produced using the Solvay
Q3: In the reaction below, _ is the
Q4: Oxidation refers to _
A)an increase in oxidation
Q6: Oxidation is the _
A)gain of electrons.
B)loss of
Q7: Where in the periodic table do you
Q8: When aluminum metal is obtained from aluminum
Q9: Reduction refers to _
A)a decrease in oxidation
Q10: What is the oxidation number of chromium
Q11: The following reaction occurs in basic solution.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents