You have a job as a summer intern in an orange juice processing plant. Part of your job is to determine the amount of vitamin C in a given quantity of orange juice. To make this determination, you titrate the orange juice with I3-, the triiodide ion. To make sure you know what you are doing, your supervisor asks you how many electrons are transferred in the reaction. The reaction describing the titration is given below. What is your response?
ascorbate 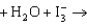 dehydroascorbate + 2H+ + 3I-
dehydroascorbate + 2H+ + 3I-
A) 1
B) 2
C) 4
D) 0
E) It is impossible to tell.
Correct Answer:
Verified
Q28: The electrodes on batteries are labeled +
Q29: The diagram below represents a voltaic cell.
Q30: Which statement regarding voltaic cells is not
Q31: The diagram below represents a voltaic cell.
Q32: Which cell diagram is correct for the
Q34: A voltaic cell is constructed based on
Q35: Consider an electrochemical cell with a Zn
Q36: Which statement is not correct for a
Q37: For the following reaction, which statement A-D
Q38: The electrodes on batteries are labeled +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents