Consider an electrochemical cell with a Zn electrode in ZnSO4(aq) and a Cu electrode in CuSO4(aq) . The overall chemical reaction is 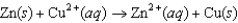
Which one of the following statements is correct?
A) Copper is oxidized at the anode.
B) One mole of electrons is transferred in this reaction.
C) Zinc is reduced at the cathode.
D) Copper ions are reduced at the cathode.
E) Electrons travel from the Cu electrode to the Zn electrode.
Correct Answer:
Verified
Q30: Which statement regarding voltaic cells is not
Q31: The diagram below represents a voltaic cell.
Q32: Which cell diagram is correct for the
Q33: You have a job as a summer
Q34: A voltaic cell is constructed based on
Q36: Which statement is not correct for a
Q37: For the following reaction, which statement A-D
Q38: The electrodes on batteries are labeled +
Q39: Which cell diagram is correct for this
Q40: A voltaic cell is constructed based on
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents