Use the table of standard reduction potentials below to identify the metal or metal ion that is the strongest reducing agent. 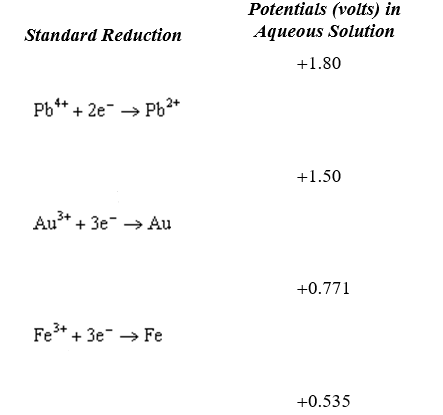
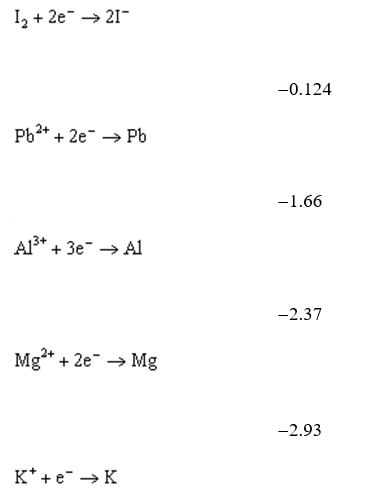
A) Pb4+
B) Pb2+
C) K+
D) K
E) Al
Correct Answer:
Verified
Q52: Using the following data, determine the
Q53: Which statement about a voltaic cell is
Q54: Based on the information in the table
Q55: Consider the following standard reduction potentials. Reduction
Q56: The diagram below represents a voltaic cell.
Q58: Using the following data, determine the standard
Q59: Based on the information in the table
Q60: The diagram below represents a voltaic cell.
Q61: If the free-energy change of the following
Q62: The magnitude of the charge on
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents