The diagram below represents a voltaic cell. What is the balanced electrochemical reaction represented by this cell? Al(s) |Al3+(1.0 M) ||Cu2+(1.0 M) |Cu(s)
A) 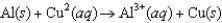
B) 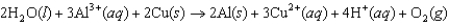
C) 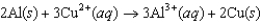
D) 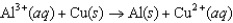
E) 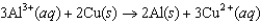
Correct Answer:
Verified
Q55: Consider the following standard reduction potentials. Reduction
Q56: The diagram below represents a voltaic cell.
Q57: Use the table of standard reduction potentials
Q58: Using the following data, determine the standard
Q59: Based on the information in the table
Q61: If the free-energy change of the following
Q62: The magnitude of the charge on
Q63: A concentration cell is constructed by using
Q64: An electrochemical cell with a standard
Q65: The spontaneous redox reaction in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents