A concentration cell is constructed by using the same half-reaction for both the cathode and anode. What is the value of standard cell potential, 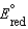 , for a concentration cell that combines a silver anode in contact with 0.10 M silver nitrate and a silver cathode in contact with 0.00003 M silver nitrate? (
, for a concentration cell that combines a silver anode in contact with 0.10 M silver nitrate and a silver cathode in contact with 0.00003 M silver nitrate? ( 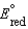 = +0.80 V for Ag/Ag+)
= +0.80 V for Ag/Ag+)
A) (-0.21 V)
B) 0.00 V
C) (+0.80 V)
D) (-0.80 V)
E) (+0.21 V)
Correct Answer:
Verified
Q58: Using the following data, determine the standard
Q59: Based on the information in the table
Q60: The diagram below represents a voltaic cell.
Q61: If the free-energy change of the following
Q62: The magnitude of the charge on
Q64: An electrochemical cell with a standard
Q65: The spontaneous redox reaction in a
Q66: The work involved in moving exactly 1
Q67: If the potential of a voltaic cell
Q68: Copper is oxidized by nitric acid. If
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents