The oxidation of hydrogen by oxygen is one of the most-used reactions in fuel-cell technology. The overall reaction, which is given below, has a G value of -474 kJ/mol. What is the standard cell potential for this fuel cell? 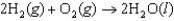
A) 2.46 V
B) 4.91 V
C) 1.23 V
D) 3.05 V
E) 1.50 V
Correct Answer:
Verified
Q127: How do fuel cells differ from traditional
Q128: If in using a lead-acid battery to
Q129: Applying a current to a rechargeable battery
Q130: How many kilograms of aluminum metal
Q131: Which one of the following statements about
Q133: Copper metal is purified by electrolysis. How
Q134: Bubbles will form on wires attached to
Q135: Cathodic protection occurs when iron is _
A)attached
Q136: The oxidation of methanol, as described
Q137: Aluminum metal is refined in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents