The oxidation of methanol, as described by the equation below, has a G value of -937.9 kJ/mol. What is the standard cell potential for a methanol fuel cell? 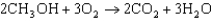
A) 0.405 V
B) 9.72 V
C) 0.810 V
D) (-2.43 V)
E) (-9.72 V)
Correct Answer:
Verified
Q131: Which one of the following statements about
Q132: The oxidation of hydrogen by oxygen
Q133: Copper metal is purified by electrolysis. How
Q134: Bubbles will form on wires attached to
Q135: Cathodic protection occurs when iron is _
A)attached
Q137: Aluminum metal is refined in a
Q138: The oxidation of hydrogen by oxygen
Q139: Which of the following is not an
Q140: Which statement regarding battery-powered electric cars is
Q141: What kind of chemical reaction occurs at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents