Given the following data relevant to the combustion of ethanol, determine the free energy of formation for liquid ethanol, C2H5OH. 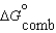 (C2H5OH, l) -930.7 kJ/mol
(C2H5OH, l) -930.7 kJ/mol 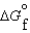 (CO2, g) -394.4 kJ/mol
(CO2, g) -394.4 kJ/mol 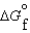 (H2O, g) -105.6 kJ/mol
(H2O, g) -105.6 kJ/mol
A) (-1,640.3 kJ/mol)
B) (-244.2 kJ/mol)
C) (-174.9 kJ/mol)
D) (+174.9 kJ/mol)
E) (+244.2 kJ/mol)
Correct Answer:
Verified
Q56: Which of the following is in the
Q57: The standard molar entropy of lead(II) bromide
Q58: What is the standard entropy change when
Q59: Determine
Q60: If 3.500 g of Ni are reacted
Q62: Hydrogen reacts with nitrogen to form
Q63: Alcohols for use as biofuels can
Q64: Consider substances that exist as liquids under
Q65: Which of the following is/are true
Q66: Determine ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents