Consider substances that exist as liquids under standard state conditions. What must be the relationship between the enthalpy and free energy of formation for the liquid and the gaseous form of such a substance? (Read < as more negative and > as less negative.)
A) 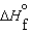 (l ) <
(l ) < 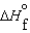 (g) and
(g) and 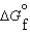 (l ) <
(l ) < 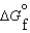 (g)
(g)
B) 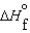 (l ) <
(l ) <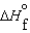 (g) and
(g) and 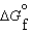 (l ) >
(l ) > 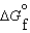 (g)
(g)
C) 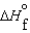 (l ) >
(l ) > 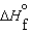 (g) and
(g) and 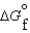 (l ) >
(l ) > 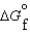 (g)
(g)
D) 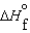 (l ) >
(l ) > 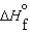 (g) and
(g) and 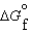 (l ) <
(l ) < 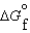 (g)
(g)
E) No strict relationship between these values applies.
Correct Answer:
Verified
Q59: Determine
Q60: If 3.500 g of Ni are reacted
Q61: Given the following data relevant to the
Q62: Hydrogen reacts with nitrogen to form
Q63: Alcohols for use as biofuels can
Q65: Which of the following is/are true
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents