If a reaction 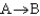 has
has 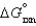 > 0, then when the reaction is at equilibrium there will be ________
> 0, then when the reaction is at equilibrium there will be ________
A) absolutely no B that is formed.
B) smaller quantities of B and larger quantities of A.
C) equal quantities of A and B.
D) large quantities of B and smaller quantities of A.
E) absolutely no A remaining.
Correct Answer:
Verified
Q97: When a solution of DNA in
Q98: For the following endothermic reaction, predict under
Q99: The dissolution of ammonium nitrate in water
Q100: A reaction with a low enthalpy
Q101: If
Q103: If for a given chemical reaction
Q104: Suppose
Q105: For a particular hypothetical reaction,
Q106: A perturbation or stress to a chemical
Q107: If
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents