For the following endothermic reaction, predict under which conditions the reaction will be spontaneous. 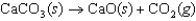
A) The reaction is always spontaneous.
B) The reaction is never spontaneous.
C) The reaction is spontaneous at high temperatures.
D) The reaction is spontaneous at low temperatures.
E) Insufficient data is provided to answer this question.
Correct Answer:
Verified
Q93: The entropy of vaporization of water is
Q94: The enthalpy and entropy of vaporization
Q95: Calcium sulfate is a desiccant used
Q96: At what temperature does the Fe(s)
Q97: When a solution of DNA in
Q99: The dissolution of ammonium nitrate in water
Q100: A reaction with a low enthalpy
Q101: If
Q102: If a reaction Q103: If for a given chemical reaction![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents