In a biochemical reaction, A + B C with 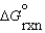 = 30 kJ/mol. Which of the following reactions might be effectively coupled to this reaction so that it becomes more spontaneous?
= 30 kJ/mol. Which of the following reactions might be effectively coupled to this reaction so that it becomes more spontaneous?
I.C + D B + E 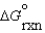 = - 40 kJ/mol
= - 40 kJ/mol
II.C + D B + E 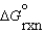 = + 40 kJ/mol
= + 40 kJ/mol
A) I only
B) II only
C) I or II
D) Neither I nor II can increase spontaneity.
E) No coupling is required, because the reaction is already spontaneous.
Correct Answer:
Verified
Q151: Boltzmann derived the relationship S = k
Q152: Which statement is true of the ideal
Q153: What type of motion is depicted in
Q154: Noxious NO gas can form from
Q155: Which of the following states of motion
Q157: Which of the following must be true
Q158: Give an example of a spontaneous and
Q159: Which of the following is the best
Q160: Which statement about the reaction below, where
Q161: Hydrogen iodide can theoretically be made
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents