Noxious NO gas can form from N2 and O2 gases in automobile engines at high temperatures. If the value of the equilibrium constant, Kc, for this reaction at 25 C is 1.95 10-31, what is the value at 2,000 C? 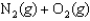
Thermodynamic Properties of Nitrogen Monoxide 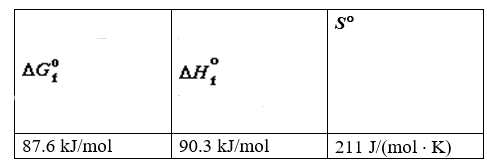
A) 2.8 103
B) 3.7 102
C) 6.9 10-2
D) 6.2 10-4
E) 1.7 10-6
Correct Answer:
Verified
Q149: The standard entropy of nitrogen gas
Q150: A chemist is planning to study
Q151: Boltzmann derived the relationship S = k
Q152: Which statement is true of the ideal
Q153: What type of motion is depicted in
Q155: Which of the following states of motion
Q156: In a biochemical reaction, A +
Q157: Which of the following must be true
Q158: Give an example of a spontaneous and
Q159: Which of the following is the best
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents