In an experiment, 1.00 atm of N2(g) in a 10.0 L container at 25
C was reacted under standard state conditions with a stoichiometric quantity of H2(g) to form ammonia: 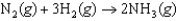 .
.
What is the entropy change for the reaction? 
Correct Answer:
Verified
Q168: The standard molar enthalpy of fusion
Q169: Carbon monoxide has a very weak dipole
Q170: Nitrogen monoxide molecules can react to
Q171: Determine the normal melting point of benzoic
Q172: Determine the value of
Q174: What is the standard entropy change when
Q175: Hydrochloric acid (HCl) reacts with sodium
Q176: The heat of fusion for water
Q177: For a particular hypothetical reaction,
Q178: Given the following data, determine the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents