Which of the listed perturbations would change the value of the equilibrium constant for the following reaction? List those that do as a sequence of letters, for example, ACE.
NH4CO2NH2(s) 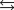 2NH3(g) + CO2(g)
2NH3(g) + CO2(g)
A) Increasing the quantity of NH4CO2NH2(s)
B) Removing CO2(g)
C) Increasing the total pressure by adding argon gas
D) Increasing the volume of the container
E) Increasing the temperature
Correct Answer:
Verified
Q176: The heat of fusion for water
Q177: For a particular hypothetical reaction,
Q178: Given the following data, determine the
Q179: Name and state the first three laws
Q180: Rank the following compounds from smallest
Q181: The standard entropy of diamond is 2.4
Q182: The standard entropy of N2(g) is 191.5
Q183: Calculate the equilibrium constant at 500
Q184: Values for
Q186: What is a microstate and how are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents