Calculate the equilibrium constant at 500 K for the following reaction given the data below:
N2(g) + 3H2(g) 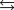 2NH3(g)
2NH3(g)
Kc (298 K) = 6.1 105 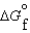 (NH3, g) = -16.5 kJ/mol
(NH3, g) = -16.5 kJ/mol 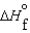 (NH3, g) = -46.1 kJ/mol
(NH3, g) = -46.1 kJ/mol
Correct Answer:
Verified
Q176: The heat of fusion for water
Q177: For a particular hypothetical reaction,
Q178: Given the following data, determine the
Q179: Name and state the first three laws
Q180: Rank the following compounds from smallest
Q181: The standard entropy of diamond is 2.4
Q182: The standard entropy of N2(g) is 191.5
Q184: Values for
Q185: Which of the listed perturbations would change
Q186: What is a microstate and how are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents