Calculate the equilibrium concentration of Zn2+(aq) in a solution that is 0.0125 M Zn(NO3) 2 and 0.600 M NH3.
Given: Zn2+(aq) + 4NH3(aq) 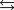 Zn(NH3) 42+(aq) Kf = 2.9 109.
Zn(NH3) 42+(aq) Kf = 2.9 109.
A) 7.8 10-12 M
B) 4.7 10-11 M
C) 1.5 10-8 M
D) 2.5 10-9 M
E) 2.9 109 M
Correct Answer:
Verified
Q59: Which of the following species is a
Q60: A Lewis acid is any species capable
Q61: The bond between a metal cation and
Q62: Hydrated transition metal ions produce solutions that
Q63: Determine the molar concentration of Ag+(aq)
Q65: Which of the following compounds contains a
Q66: When Ni(NH3)6Cl2 dissolves in water, which of
Q67: Which of the following species is most
Q68: What is the equilibrium concentration of
Q69: Which of the following species is least
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents