What is the equilibrium concentration of Cu+(aq) in a solution that is 0.0200 M solution in CuNO3 and 0.450 M HCl(aq) ? Given: Cu+(aq) + 3Cl-(aq) 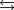 CuCl32- Kf = 5 105.
CuCl32- Kf = 5 105.
A) 1.0 10-7 M
B) 6.7 10-7 M
C) 4.4 10-7 M
D) 5.0 10-7 M
E) 5.0 105 M
Correct Answer:
Verified
Q63: Determine the molar concentration of Ag+(aq)
Q64: Calculate the equilibrium concentration of Zn2+(aq)
Q65: Which of the following compounds contains a
Q66: When Ni(NH3)6Cl2 dissolves in water, which of
Q67: Which of the following species is most
Q69: Which of the following species is least
Q70: What is the molar concentration of
Q71: Which statement A-D regarding acids and bases
Q72: Which statement A-D regarding a complex ion
Q73: Many metal cations produce aqueous solutions that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents