Vitamin C, which is ascorbic acid, is a diprotic acid with 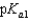 = 5.00 and
= 5.00 and 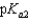 = 11.3. What is the pH of a 0.125 M solution of ascorbic acid?
= 11.3. What is the pH of a 0.125 M solution of ascorbic acid?
A) 2.95
B) 3.05
C) 5.00
D) 6.10
E) 3.54
Correct Answer:
Verified
Q73: Identify the weakest and strongest acids in
Q74: Which ending to the statement is not
Q75: The pH of vinegar is 2.4, and
Q76: The pH of an ammonia solution is
Q77: Identify the weakest and strongest acids in
Q79: The degree of ionization _
A)increases with increasing
Q80: Identify the weakest and strongest acids in
Q81: Aqueous solutions of _ are acidic.
A)NaF
B)CsCN
C)MgCl2
D)NH4I
E)KI
Q82: Which of the following salts produce(s) a
Q83: Which of the following salts produce(s) a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents